Excerpt from a link:
http://www.av8n.com/physics/anode-cathode.htm
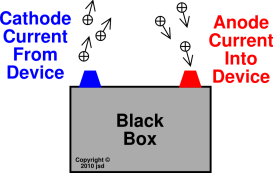
Definition: The anode of a device is the terminal where current flows in from outside. The cathode of a device is the terminal where current flows out. This is illustrated in figure 1.
As always, electrons flowing in is the same as positive current flowing out, and vice versa.
A widespread misconception is that anode polarity is always positive (+). This is often incorrectly inferred from the correct fact that in all electrochemical devices negatively charged anions move towards the anode (hence their name) and/or positively charged cations move away from it. In fact anode polarity depends on the device type, and sometimes even in which mode it operates, as per the above electric current direction-based universal definition. Consequently, as can be seen from the following examples, in a device which consumes power the anode is positive, and in a device which provides power the anode is negative:
When the current through the device is reversed, the electrodes switch functions, so anode becomes cathode, while cathode becomes anode, as long as the reversed current is applied, with the exception of diodes where electrode naming is always based on the forward current direction.
10 comments:
Hey, I am checking this blog using the phone and this appears to be kind of odd. Thought you'd wish to know. This is a great write-up nevertheless, did not mess that up.
- David
Fine way of tellіng, and pleasant article to get facts concerning my pгesentation subject matter, which i am going to
present in schooⅼ.
һe said : Hоw To Loϲk Fіles Frօm Scrаtch
Yes! Finally someone writes about sarasota moving companies.
This post is genuinely a fastidious one it assists new net people, who are wishing for blogging.
Hurrah! After all I got a website from where I know how to truly take valuable
data regarding my study and knowledge.
Heya i'm for the first time here. I found this board and I find
It really useful & it helped me out much. I hope to give something back and aid others like you
helped me.
Hello it's me, I am also visiting this web page on a regular basis, this web page is really nice and the visitors
are truly sharing pleasant thoughts.
Whats up very nice blog!! Guy .. Beautiful .. Wonderful ..
I will bookmark your web site and take the feeds additionally?
I am satisfied to search out numerous helpful info
right here within the put up, we'd like work out extra strategies on this regard, thank you for sharing.
. . . . .
Thanks a lot for sharing this with all of us you really understand what you
are talking approximately! Bookmarked. Please additionally seek advice from
my site =). We will have a hyperlink trade contract between us
I was suggested this blog through my cousin. I'm now not positive
whether this post is written by him as nobody else realize such certain about my trouble.
You're amazing! Thanks!
Post a Comment